When you think about pollution sources, you might imagine dirty power plants, toxic chemical factories emitting dirty smoke, or gasoline engines. You might not think of farms. But despite the pristine green fields, agriculture is actually a major source of pollution in the United States. Rampant overuse of synthetic nitrogen fertilizer is creating a multi-pronged pollution crisis. Farmers are caught in a system that forces their hand to apply about twice as much fertilizer as their crops can use. Fertilizer overkill threatens the nation’s water resources and the people who rely on them, damages farm soils, spews surprising amounts of a potent heat-trapping gas into our atmosphere, and is literally disrupting one of the planet’s major natural cycles.
Crucially, this system harms farmers themselves—raising their input costs, degrading their soil over time, and locking them into cropping patterns that are hard to escape.
Fertilizer overuse is accelerating the climate crisis
The problem is not that farmers use fertilizer—plants need it to grow. Rather, the problem is how much farmers use and how much land our current system devotes to continuous production of nitrogen-intensive crops.
Research shows that as much as 50% of applied nitrogen fertilizer is in excess of what crops need and is unused by plants. A portion of the excess unused fertilizer gets converted into gases like nitrous oxide (N2O) by soil microbes and released into the environment. Nitrous oxide is a potent heat-trapping gas: 265 times more powerful than carbon dioxide when it comes to its warming potential. In 2022, N2O accounted for 6% of all heat-trapping emissions in the United States.
Agriculture is the United States’ undisputed largest source of N2O, with agricultural soil management responsible for as much as 75% of the country’s N2O emissions. N2O also comes from other sources like burning fossil fuels, manufacturing nitric acid, and manufacturing fertilizer. In agricultural soils, use of synthetic nitrogen fertilizers accounts for the majority of N2O emissions. To reduce these emissions, fertilizer overuse must come down. But it’s important to recognize that farmers often shoulder the financial and environmental burden of trying to manage risks in a system that incentivizes overapplication.
Fertilizer overuse pollutes our water and degrades our soil
Nitrogen overuse wreaks havoc in the environment because the runoff from unused fertilizer contains a vast amount of reactive nitrogen in the form of nitrates. When washed into lakes and streams, this reactive nitrogen helps algae multiply very quickly and create algal blooms. Algal blooms consume dissolved oxygen in the water, creating low- to no-oxygen areas in aquatic ecosystems called “dead zones” where nothing can survive. Farmers themselves are often frustrated by these outcomes, knowing that the fertilizer they paid for is literally washing away and causing damage downstream.
The impacts of nitrogen runoff reach far and wide and can harm aquatic ecosystems thousands of miles away. The best example of this is the dead zone in the Gulf of Mexico, which returns every summer and stretches thousands of miles. In 2025, the National Oceanic and Atmospheric Administration reported the size of this dead zone at 4,402 square miles—roughly the same area as that of Connecticut. Nitrogen fertilizer runoff from the Midwest is one of the major contributors to the Gulf dead zone, estimated to cost $2.4 billion in lost livelihoods.
Nitrogen in the form of nitrate also leaches into groundwater sources and can end up contaminating drinking water supplies. Nitrate contamination is a widely reported problem in agriculture-heavy states like Illinois, Iowa, Minnesota, and Wisconsin. Exposure to nitrate is linked to a variety of serious health problems, as my colleague UCS Research Director for the Food & Environment program Stacy Woods writes. This means farming families—who are often on private wells—can be among the first harmed when nitrogen pollution reaches groundwater. Fertilizer overuse also harms the soil itself.
High doses of synthetic nitrogen acidify soils and disrupt the microbial communities that make nutrients naturally available to plants. Over time, this reduces soil biodiversity, suppresses beneficial fungi, and even harms earthworm populations that keep soils aerated and fertile. Instead of building living, resilient soils that are like sponges, heavy fertilizer use can leave land more degraded and make soils cement-like, dependent on ever-higher chemical inputs to provide the required plant nutrition. This soil degradation hurts farmers’ long-term profitability, trapping them in a cycle of needing ever more fertilizer just to maintain yields.
What is the nitrogen cycle?
Nitrogen is a classic example of something that’s good—essential for life on Earth—in small quantities, but you can have too much of a good thing. Natural biogeochemical cycles are slow processes. It takes microbes time to convert nitrogen and make it available for plants. But human-induced nitrogen cycle disruption has vastly altered how nitrogen moves and behaves in the environment, and created several short cuts that make a vast trove of reactive nitrogen ready for plant uptake.
To understand why we need fertilizers, we first must understand how nitrogen moves in the environment through a series of biogeochemical processes collectively called the nitrogen cycle.
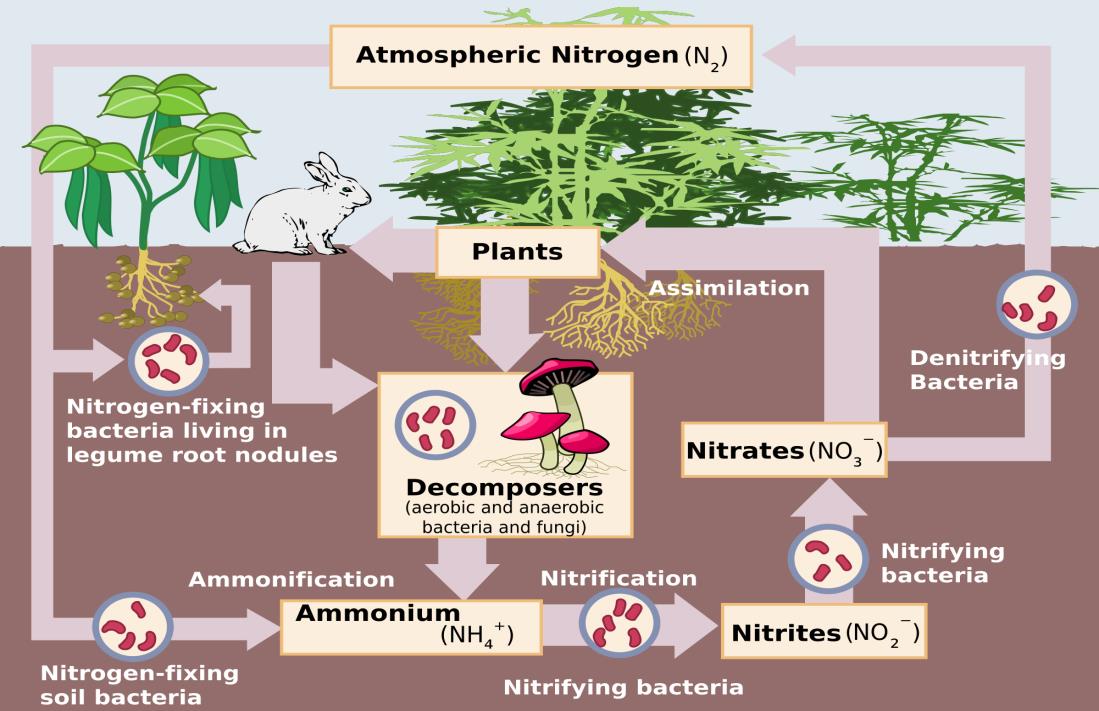
As I mentioned earlier, the most abundant form of nitrogen is atmospheric gaseous nitrogen (N2), which plants are unable to absorb. For nitrogen to become available for plants to use, it has to be converted into chemical forms like ammonium, nitrates, and nitrites that can then be absorbed through plant roots. This process is called nitrogen fixation.
Nitrogen fixation: The first step of the nitrogen cycle involves nitrogen reacting with hydrogen to form ammonia. Ammonia (NH3) is an intermediate form of nitrogen that can then be converted into nitrates and nitrites. Gaseous nitrogen is an extremely stable molecule with strong bonds, and large amounts of energy are required to break this bond and react with hydrogen to form ammonia. In nature, this energy comes from lightning, and also occurs naturally in leguminous plants which have a mutually beneficial (symbiotic) relationship with Rhizobium soil bacteria that transforms N2 into ammonium for plant uptake. In the human-derived fertilizer manufacturing process, this process is shortcut and the energy comes from the burning of fossil fuels in what is known as the Haber Bosch process
Nitrification: The next step in the nitrogen cycle is the conversion of ammonia into reactive forms of nitrogen like nitrites and nitrates. This process is called nitrification and is almost exclusively performed by various microbes in the soil. Microbes convert ammonia into forms like nitrates and nitrites that can be easily absorbed by plant roots. Plants then use these nitrates and nitrates for essential functions like synthesizing protein.
Denitrification: Nitrates that remain behind in the soil after plants have absorbed what they need are then converted back into N2 and nitrous oxide (N2O) by microbes through a process called denitrification. This allows for excess plant-available nitrogen in the soil to be released back into the atmosphere, maintaining a healthy level in the soil.
Ammonification: A second type of nitrogen conversion occurs when plants or other organisms excrete waste or die. Plant and animal tissues contain organic nitrogen in the form of proteins, amino acids, hormones, and DNA. Microbes in the soil help break down the tissue and convert the organic nitrogen to be released back into nature as ammonia. This process is known as ammonification.
Industrial agriculture has hijacked the natural nitrogen cycle
Today’s agricultural systems focus on growing vast acreages of nitrogen-hungry commodity crops like corn and soy in astonishing density—an amount of land that by itself drives enormous fertilizer demand, even if every acre were managed efficiently. These commodity crops require vast amounts of nitrogen (along with other nutrients like phosphorus and potassium) to support crop development and yield.
This is why fertilizers are added: to supplement the nitrogen in nature that plants can quickly absorb. Human activities like the use of synthetic fertilizers, livestock farming, and burning fossil fuels (with the associated emissions) have disrupted and altered the way nitrogen moves in the environment. Today’s environment has been overloaded with reactive nitrogen, which has serious implications for ecosystems, polluting air and water, and harming human health. Farmers are often acutely aware of these impacts, but deviating from the dominant system can bring real financial penalties. They haven’t chosen this system—policy, market concentration, and industry influence channel them toward high-input, high-volume production, even when it undermines long-term soil health and profitability.
What is the way forward in reducing nitrogen pollution?
The overreliance on costly agrichemical inputs and overdependence on fossil fuel fertilizers is a major driver of the climate crisis. Much of today’s fertilizer demand comes from how and where it is used—that enormous acreage devoted to continuous production of nitrogen-hungry commodity crops, especially corn. Reducing this acreage will require reforming market-distorting subsidies, particularly federally subsidized crop insurance, which rewards cultivation of nitrogen-intensive commodity crops and encourages planting, even on vulnerable land.
The first step in tackling nitrogen pollution is to prevent fertilizer overapplication. For farmers that means testing soil and applying fertilizer only where and when crops need it, and avoiding practices like blanket applications in the fall when plants do not need fertilizer and most of what is applied is wasted. Other on-farm fertilizer management practices include carefully managing irrigation of soils, since excess moisture increases N2O emissions, limiting applications to the spring, and using precise placement to maximize uptake. The logical starting point is to equip farmers with funding and technical assistance to optimize the application of fertilizers.
But neither efficiency improvements and precision, nor acreage reductions alone can deliver the scale of change required. Moving away from subsidies that encourage the overproduction of commodity crops would help transform current agricultural systems, as would investing more in US Department of Agriculture programs like the Conservation Stewardship Program and the Environmental Quality Incentives Program, which are backed by decades of science and farmer experience and can reduce reliance on costly synthetic fertilizers, thus improving soil health, protecting habitat, and improving water and air quality.
The bottom-line is clear: We need a large-scale shift to farming practices based on the science of agroecology, which treats farms like natural systems. Agroecological practices such as cover crops, buffer strips, restored wetlands, and managed grazing further help keep nutrients in place and build long-term soil health.
Our goal at UCS is to support farmers—not blame them—by helping reshape the system so they can thrive financially while reducing nitrogen pollution. There is plenty of clear scientific evidence that US agriculture needs to shift towards a model of agroecology for a healthier environment and climate.
To induce a system-wide transition to agricultural practices that build soil health and allow farmers to reduce costly inputs, UCS is tackling the problem of fertilizers. We aim to shine a light on how corporate entities keep farmers hooked on this treadmill of costly fertilizer and pesticide inputs, ultimately reducing their overreliance on synthetic nitrogen fertilizers. We continue to advocate for a transformational food and farming system that is more equitable and resilient, and that works for farmers, farmworkers, consumers, and our environment.

